29+ Why Are Noble Gasses Unreactive
Their outer shell is full meaning it is difficult for them to give or take. And as their outer shell is full indicating it is complicated.

Noble Gases Periodic Table Chemtalk
The reason as to why these elements are called noble is because.

. He is duplet configuration Ne is octet configuration hence they do not readily bond with other. Their uses depend on their inertness low density and. June 30th 2022 Generally atoms react and complete their octet to become stable.
By PSIBERG Team Last Updated. They show trends in their physical properties. Web Because they contain a full valence shell of electrons.
Web Generally noble gases are very unreactive. Web Noble gas elements of G-18--edit edit sourceThe noble gases are in Group 18 8AThey are helium neon argon krypton xenon and radonThey were once. Web Why are noble gases unreactive.
Web Noble gases are so unreactive because of the number of electrons present in the outermost shell of their atoms. Web Noble gas atoms are unreactive because they have a fully filled valence shell eg. Because of this configuration they are i difficult to reduce electrons.
Web The group 0 elements the noble gases are all unreactive non-metal gases. Web Why are noble gases so unreactive. I understand that it has to do with electron shielding prospective electrons that could join the atom or leave and the.
Web Why are noble gases unreactive What Are Noble Gases. Noble gases contain a full valence of electrons. This is due to the number of electrons in the outer shell of their atoms.
Web 0000 - Are noble gases largely unreactive0040 - Which is the most unreactive gas0108 - What are the uses of noble gas0141 - Why is Krypton so unreacti. Because of this configuration they are i. Web Answer Noble gases are unreactive as their valence shells or outermost electron shells are completely filled.
Noble gases are six monoatomic gaseous elements found in nature that share similar chemical properties. Web Noble gases contain a full valence of electrons. Web This makes noble gases unreactive.
The elements that make up the last group in the. Because of this configuration they are i difficult to reduce electrons must enter the next valence shell and ii difficult to oxidize.
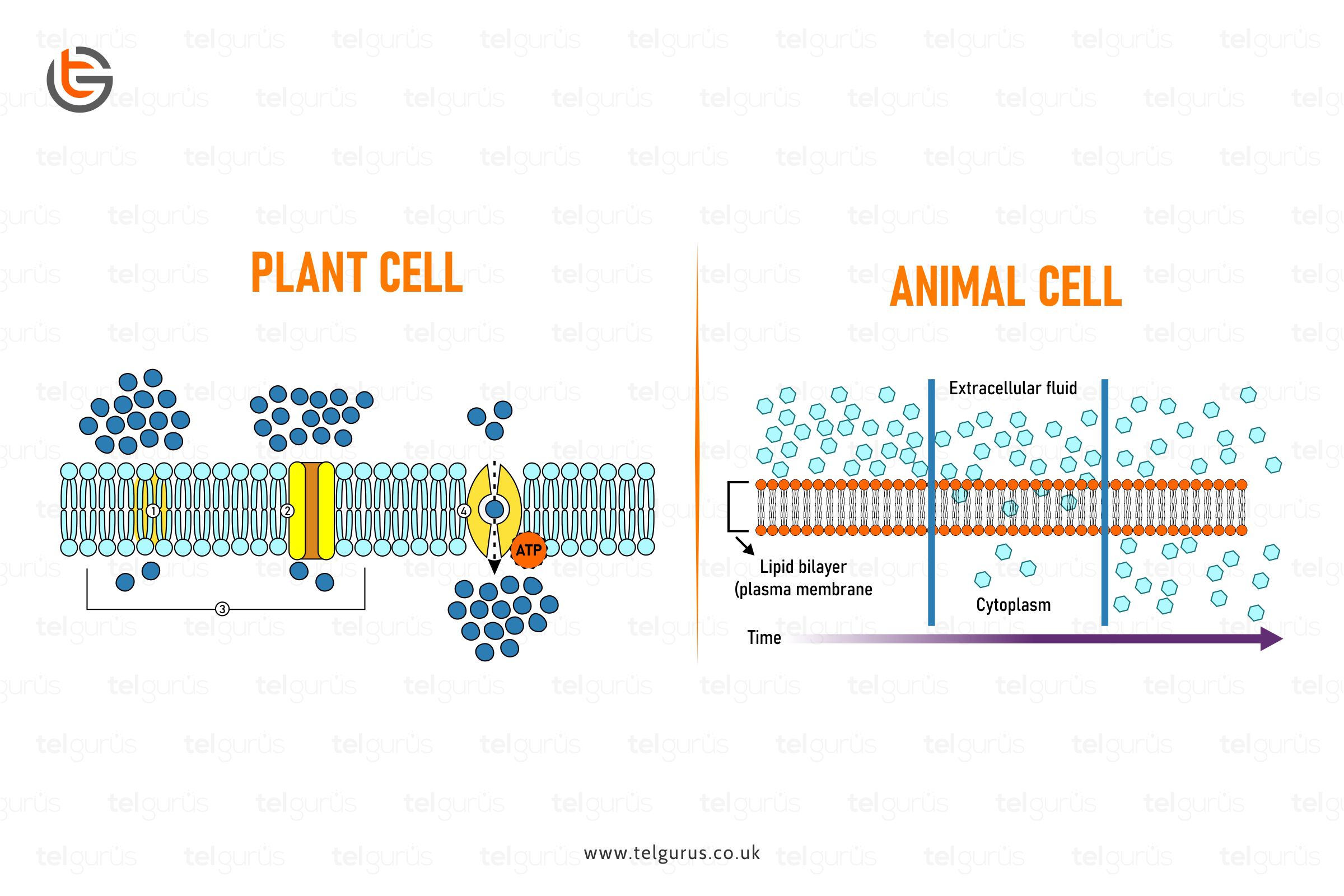
Why Are Noble Gases So Unreactive Science Questions Tel Gurus
Why Aren T Noble Gasses Group Viii Reactive Quora

Chemical Properties Of The Noble Gases Group 0 The Noble Gases Edexcel Gcse Combined Science Revision Edexcel Bbc Bitesize

Advances In Organometallic Chemistry Volume 40 Pdf Free Download

What Is The Reason Behind Some Noble Gases Such As Argon To React Exceptionally Quora

Why Are Noble Gases So Unreactive Science Questions Tel Gurus

Advances In Organometallic Chemistry Volume 40 Pdf Free Download

12 Cdrtgkiui Pdf

Encyclopedia Of Electrochemistry Volume 7b Inorganic Electrochemistry Encyclopedia Of Electrochemistry Pdfdrive Pdf Coordination Complex Redox

Why Do Noble Gases Show Least Reactivity Quora

Why Are Noble Gases Are Unreactive Brainly In

Noble Gases Why Noble Gases Are Not Reactive Configuration By Chemistry Page Medium
Group 18 Reactions Of Nobel Gases Chemistry Libretexts

Why Are Noble Gases Very Unreactive Quora

Why Are Noble Gases Very Unreactive Quora

The Noble Gases Are Unreactive Because They
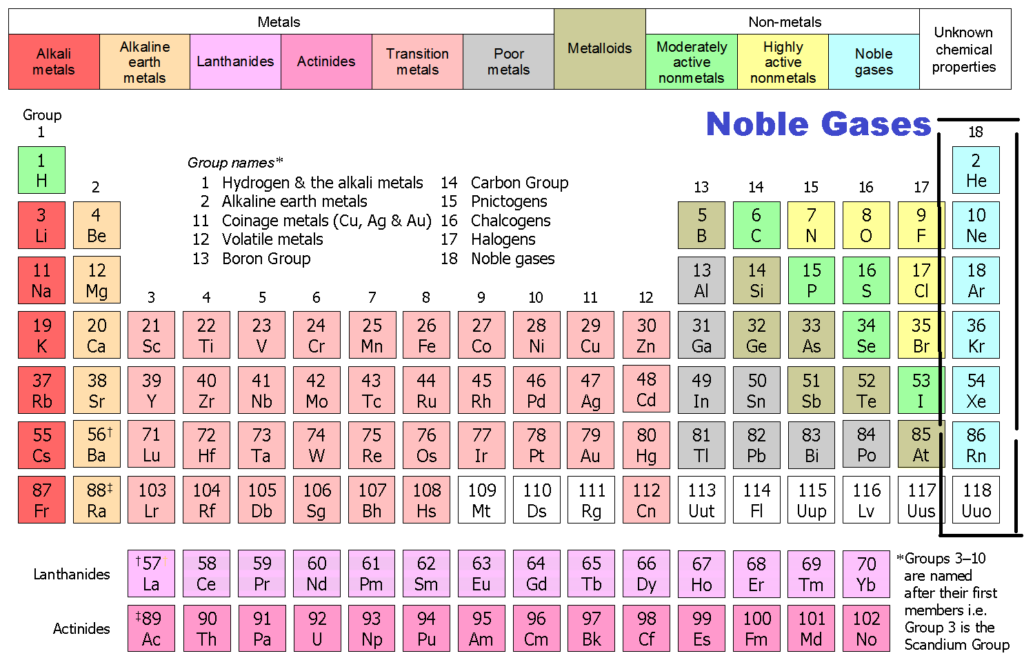
Noble Gases Why Noble Gases Are Not Reactive Configuration By Chemistry Page Medium